IEA HYPSILL
Unraveling HYdrogen SPILLover in heterogeneous catalysts for hydrogen transportationIEA HYPSILL
2024 – 2025
Contact:
French Partner :
Nuno Rocha Batalha
Australian partner:
Muxina Konarova
NEWS
Introduction
Multiple governments have developed a plan where H2 is an imperative pathway to carbon neutrality [1]. Yet, for renewable H2 to become competitive and the current targets attained, its costs must be reduced. While numerous efforts have been made to make renewable hydrogen production cost-competitive, with production expected to match fossil H2 in 2030 [2], H2 transportation and storage still stand as the “Achilles heel” of the hydrogen economy. Indeed, the cost associated with H2 transportation and storage can represent up to 3 times that of production [3].
Among the available hydrogen storage and transport solutions, Liquid Organic Hydrogen Carriers (LOHCs) are promising candidates since they allow hydrogen storage in liquid form at ambient temperature and pressure. LOHC technology uses stable liquid organic molecules, i.e., an H2-lean compound (LOHC) and an H2-rich compound (LOHC-H), which can be reversibly transformed into each other by catalytic dehydrogenation and hydrogenation reactions (Figure 1). Since the carriers are oil derivatives, they are compatible with existing liquid fuel infrastructures for storage and transport requirements. Furthermore, LOHCs are safe (high stability), have high hydrogen content (up to 7% wt. H2), reduced cost (cycle process in agreement with a circular economy), and address the low carbon paradigm of H2 transportation (high LOHC recyclability). The capacity to efficiently recycle the LOHC molecules, which is linked to the selectivity of the hydrogenation and dehydrogenation reaction, is crucial for the economic feasibility of this technology.
Nowadays, the commercial catalysts for LOHC hydrogenation and dehydrogenation are performed by noble metals, like Pt or Ru, which enable good activity (fast H2 loading and recovery) and selectivity. However, these materials are increasingly rare and expensive, and their reserves are geographically limited to a few countries. Therefore, the lack of alternative catalysts that can be used for LOHC hydrogenation and dehydrogenation with good performance (activity and selectivity) stands as one of the main barriers to the effective emergence of LOHC, enabling their widespread transport and storage of hydrogen [4].
Main objectives of research
Hydrogenation on heterogeneous catalysts proceeds through the organic compound, e.g., LOHC, and adsorption on an active metal surface, e.g., noble metal or transition metal, for the reaction to occur. However, the metallic surface represents only a tiny fraction of the overall area of the catalyst. Commonly, metallic catalysts are deposited on an inert support, e.g., Al2O3, to maximize the metallic surface area per amount of used metal, i.e., dispersion. Yet, the metal dispersion is favored by low metallic loadings. For instance, Lee et al. showed that, on Pt/Al2O3 (the commercial catalyst for LOHC dehydrogenation), platinum dispersion decreases ≈ 4 times when increasing the loading from 0.5 wt.% to 10 wt.%5. Hence, the metal loading must be kept low to minimize the use of an expensive metallic phase, leading to a catalyst whose surface is mostly inactive. This problem is particularly important when using transition metals, like Ni, as the main active phase since these are ≈100 times less activity when compared to noble metals6 and, for this reason, must be used in larger quantities.
Multiple studies have shown that, while not typically active for hydrogenation, some catalyst supports, e.g., zeolite and TiO2, can participate in the reaction when near a metal particle capable of H2 dissociation through hydrogen spillover (HSP) [7]. Hsp is defined as the migration of active hydrogen species between the metal and the catalyst support, which cannot dissociate H2. Thus, the reaction is no longer centralized on the metal surface since both support and metal are active. As a result, Hsp leads to an overall increase in activity, reducing the amount of metal needed to attain the same goal. Compared to the metal phase, the support is relatively inexpensive and can even be obtained from waste materials with an improved environmental footprint [8],[9]. Additionally, Hsp diffusion on a support is not directly linked to the metal responsible for hydrogen dissociation. Indeed, HSP has been reported on catalysts containing noble and transition metals, e.g., Ni [7]. Still, despite the potential of HSP for enhancing the catalytic performance of hydrogenation heterogeneous catalysts, its use to enhance hydrogen transportation and storage via LOHC is still unexplored.
While the effects of Hsp in heterogeneous catalysts have been reported for multiple decades, the lack of understanding of how the phenomenon operates under catalytic reaction conditions and what catalyst parameters are crucial for it to enhance catalytic performance hinders broader application. Additionally, while the benefits of Hsp under the liquid phase have been reported [10], most reports on the catalytic benefits of Hsp were done in the gas phase, which is not commonly used for LOHC processing. The lack of understanding surrounding the Hsp phenomenon is directly linked to the absence of experimental results based on well-defined model systems. This can provide clear evidence of how Hsp can be exploited for catalytic purposes [11]. Indeed, over the years, Hsp has been, more often than not, used to explain unexpected results without a supported basis [12]. Thus, using model catalysts and experimental methods that identify the crucial parameters and establish concrete evidence is essential to understanding and exploiting Hsp and, consequently, developing and exploiting this phenomenon under practical conditions.
Objectives: The primary purpose of this project is to open the path for using Hsp to facilitate hydrogen transportation and storage by enhancing the performance of LOHC hydrogenation catalysts. Such feat will be attained by:
1 – Establishing a parallel between Hsp behavior and the catalyst supports physicochemical properties.
2 – Establishing the mechanisms behind Hsp-based hydrogenation,
3 – Establishing the benefits of Hsp phenomenon for hydrogen loading on LOHC under liquid-phase conditions ( most
common condition for LOHC hydrogenation)
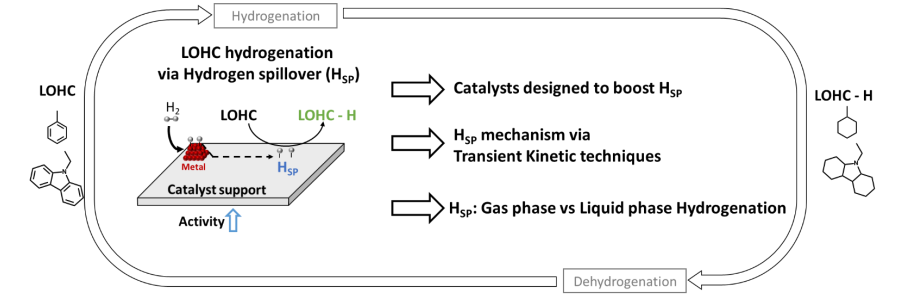
Figure 1 : Summary of the project
Institutions and laboratories involved
France
Institute des Recherches sur la Catalyse et environment de Lyon (IRCELYON, CNRS UMR 5256)
Australia
School of Chemical Engineering of the University of Queensland (UQ)
References
[1]. Pompili, B. & Le Maire, B. Stratégie nationale pour le développement de l’hydrogène décarboné en France. (2020)
[2]. IEA. Global Hydrogen Review 2022. (2022)
[3]. IEA. The Future of Hydrogen : Seizing todays opportunities. (2019).
[4]. Salman et al. Catalysis in Liquid Organic Hydrogen Storage: Recent Advances, Challenges, and Perspectives. Ind. Eng. Chem. Res. 61, 6067 (2022).
[5]. Lee et al Morphology and size of Pt on Al2O3: The role of specific metal-support interactions between Pt and Al2O3. J. Catal. 385, 204 (2020)
[6]. Kim et al. State-of-the-art Catalysts for Hydrogen Storage in Liquid Organic Hydrogen Carriers. Chem. Lett. 51, 239 (2022)
[7]. Li, M. et al. Hydrogen spillover as a promising strategy for boosting heterogeneous catalysis and hydrogen storage. Chem. Eng. J. 471, 144691 (2023)
[8]. Ahmed al. Red-mud based porous nanocatalysts for valorisation of municipal solid waste. J. Hazard. Mater 396, 122711
(2020).
[9].Bennett et al. Catalytic applications of waste derived materials. J. Mater. Chem. A 4, 3617 (2016)
[10]. Kim et al. Enhancement in the metal efficiency of Ru/TiO2 catalyst for guaiacol hydrogenation via hydrogen spillover in the
liquid phase. J. Catal. 410, 93 (2022).
[11]. Xiong, et al. Spillover in Heterogeneous Catalysis: New Insights and Opportunities. ACS Catal. 11, 3159 (2021).
[12]. Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 112, 2714 (2012).
